- Correct classification and risk class according to MDR
- Declaration of conformity according to the Machinery Directive also for sports treadmills
Dear h/p/cosmos sales and service partners,
h/p/cosmos lives up to its slogan "ahead of time" not only with innovative products and technologies, but also in the relevant regulatory areas to meet all legal, normative and regulatory requirements. h/p/cosmos has been certified according to MDD since 1998 and, since 2022, also according to MDR by a notified body. https://www.hpcosmos.com/en/contact-support/media-downloads/certificates
This not only creates trust, but also safety for patients, users and distributors of medical devices. This document from the "Regulatory Update" series is dedicated to the correct risk classification by manufacturers and highlights excerpts from the technical documentation to enable all economic actors, authorities and stakeholders involved to assess, plan and implement the responsibilities, necessary actions and risks accordingly.
At the h/p/cosmos International Distributor Meeting IDM 2025 from 6 to 9 April 2025 at Schloss Hohenkammer near Munich, we already reported on the topic of regulatory affairs that the BfArM in Bonn has confirmed classification rule 9 for treadmills, which h/p/cosmos has been using since 1998, and that this means every motorised treadmill for medical use requires a CE mark with a 4-digit identification number from a notified body.
https://www.hpcosmos.com/en/review-idm2025
DOWNLOAD
Complete document (15 pages) with detailed reasoning and recent court rulings in cases of incorrect risk classification
IMPORTANT: Motorised treadmills for physiotherapy and/or diagnostic applications must be classified in risk class IIa or IIb in accordance with Rule 9, and a notified body must be involved in certification and monitoring. The CE mark requires a 4-digit identification number from the notified body.
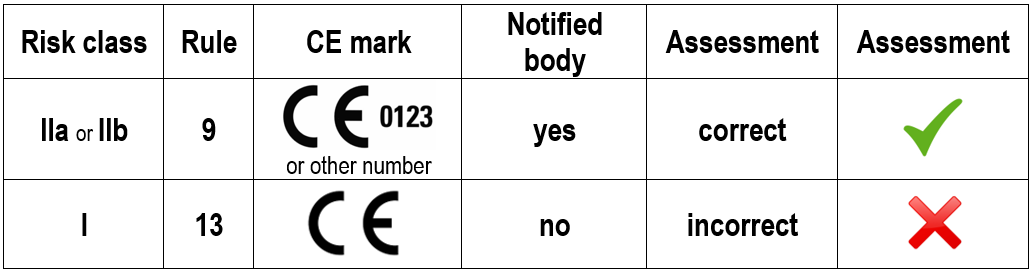 |
The MDR stipulates that incorrect classification of a medical device means that the product is not marketable and therefore may not be distributed or placed on the European market.
The applicability of Rule 9, i.e. the delivery or exchange of energy when using treadmills as medical devices, is not only based on the intended purpose, the logic and the physical and biomechanical principles of walking and running on a treadmill, but has been confirmed countless times not only by the treadmill manufacturer h/p/cosmos but also by independent authorities and/or bodies and experts from industry and science, as follows:
28.02.2025 / BfArM decision dated 28 February 2025 with reference number: 91.3.32-5634-K-0049/24
In a letter dated 11 October 2024, h/p/cosmos, as the manufacturer, submitted an application to the BfArM (Federal Institute for Drugs and Medical Devices in Germany) to decide on the classification of a motorised treadmill with an interface on the basis of Section 6 (2) MPDG (German Medical Device Law Implementation Act).
At the request of h/p/cosmos, the BfArM confirmed that, when used as intended, the treadmill exchanges energy with the patient.
1) Classification rule 9 is applicable
2) Mechanical kinetic energy is transferred/exchanged on the treadmill
3) The treadmill in question is classified as risk class IIa […]
Abbreviated excerpt from the BfArM decision of 28 February 2025 for a "simple and completely normal" treadmill without a Robowalk gait trainer, without perturbation and without high speeds, with the following specifications: motor drive, running surface 150/50 cm, speed 0 ... 22 km/h, incline angle
0 ... 25%, interface for ECG and/or spiroergometry, optional heart rate measurement via accessories (e.g. POLAR), optional adjustable handrails, treadmill without measuring function, treadmill without diagnostic function, application for physiotherapy and diagnostics.
1998 to2025 (every Jahr) / Notified body: TÜV SÜD Product Service GmbH, Munich
Certification audit and annual surveillance audit from 1998 according to MDD / MPG and since 2022 according to MDR / MPDG / ISO 13485, including review of technical documentation with confirmation of applicability of Rule 9.
https://www.hpcosmos.com/en/contact-support/media-downloads/certificates
13.09.2024 / Bavarian State Office for Health and Food Safety, Munich
Free sale certificate No. AP-2697-22-V6032-46309/12024 in accordance with Art. 60 Regulation (EU) 2017/745 in conjunction with § 10 Medical Devices Implementation Act (MPDG) in the currently applicable versions for submission to the competent authorities. ... after respective review of the EU declarations of conformity
https://www.hpcosmos.com/en/contact-support/media-downloads/certificates
Also confirmed by free sales certificates, e.g. with dates including: 8 May 2002, 28 June 2007, 2 May 2013, 18 July 2014, 1 September 2022, 29 August 2023
18.12.2023 / Paris Lodron University Salzburg / Univ.-Prof. Dr. Hermann Schwameder
Statement on energy exchange and expenditure when walking and running on a treadmill
Paris Lodron University of Salzburg, Sports and Movement Science, Head of Department, Head of Biomechanics Working Group, Schlossallee 49, 5400 Hallein-Rif, Austria download
30.11.2023 / University Hospital Halle (Saale) / Associate Professor Dr. Rene Schwesig
Assessment of energy exchange during exercise on treadmill ergometers
Research Laboratory Director, MLU Halle-Wittenberg, Faculty of Medicine, University Hospital Halle (Saale), Department of Orthopaedics, Trauma and Reconstructive Surgery, Laboratory for Experimental Orthopaedics & Sports Medicine, Ernst-Grube-Straße 40, 06120 Halle (Saale) download
13.02.2023 und 04.10.2023 / Dr. Björn Zimmermann / Sports scientist and authorised signatory at h/p/cosmos
Dr. Zimmermann has also created a PDF and a short video on the topic of energy transfer from electrically powered treadmills to the human body.
download PDF
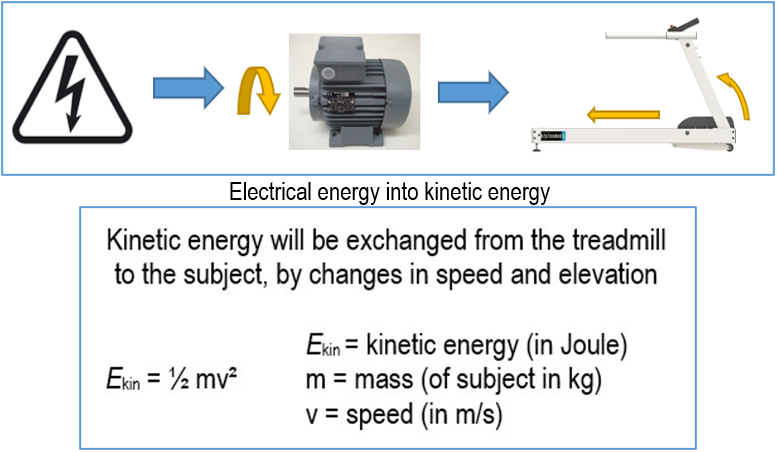
20231004_Energieuebertrag_Laufband_Geschwindigkeit_Steigung.mp4
8. April 2011 / Government of Upper Bavaria – Munich Trade Supervisory Office
[…] "The classification of medical devices follows the basic principle that the higher the potential risk of adverse effects on the human body, the higher the medical device is classified in the corresponding class. According to the recommendations of the EUROM VI and F+O working groups, based on the usual applications of medical devices, treadmill ergometers are assigned to class IIa in the list of classification examples in the "Ergometry" section.
Notwithstanding this classification, taking into account Section II No. 2.5 Annex IX Classification Criteria 93/42/EEC and the potential risk to patients described by you within the meaning of Rule 10 — active diagnostic products specifically intended for monitoring vital physiological parameters and where the nature of the change could lead to an immediate risk (deliberately induced cardiac dysfunction) to a patient — your classification under IIb is recommended. […]
The BfArM does not examine product groups, but always examines each product on a case-by-case basis, taking into account the respective intended purpose and other performance data. The applicability of Rule 9 and the fact that mechanical kinetic energy is transferred/exchanged on the treadmill has now also been confirmed by the BfArM, among others. Ergo, h/p/cosmos assumes that Rule 9 applies to all motorised treadmills because the basic laws of physics and biomechanical principles of walking and running on a treadmill are identical regardless of the manufacturer and model.
Motorised treadmill as an active medical device for physiotherapy and/or diagnostic use = Class IIa or IIb
MDR Article 2 Definitions:
(12) ‘intended purpose’ means the use for which a device is intended according to the data supplied by the manufacturer on the label, in the instructions for use or in promotional or sales materials or statements and as specified by the manufacturer in the clinical evaluation;
[...]
Not only h/p/cosmos, but also a number of other treadmill manufacturers comply with the legal and regulatory guidelines. Unfortunately, however, not all of them do. At h/p/cosmos, the test reports/verification documentation for compliance with the EU Machinery Directive/Machinery Regulation alone run to 30 pages. Here are just a few examples from the latest technical documentation from h/p/cosmos:
|
|
Test report / verification documentation |
Creator |
Pages |
1 |
Classification protocol according to conformity assessment procedure
|
h/p/cosmos |
24 |
2 |
Essential requirements according to Machinery Directive 2006/42/EC and EU
|
h/p/cosmos |
30 |
3 |
Basic safety and performance requirements according to MDR (EU) 2017/745 GSPR general safety and performance requirements
|
h/p/cosmos |
58 |
4 |
Engineering Testplan
|
h/p/cosmos |
18 |
5 |
Device specifications for models in this device family
|
h/p/cosmos |
40 |
| 6
|
Test report on mechanical safety of stationary training equipment/treadmills in accordance with EN ISO 20957-1/EN 957-6
|
DAkkS accredited and independent testing laboratory |
29 |
7 |
Risk management file for all h/p/cosmos treadmill families in accordance with DIN EN ISO14971, including plan, residual risk and report
|
h/p/cosmos |
1579 |
8 |
Risk management cybersecurity h/p/cosmos devices
|
h/p/cosmos |
94 |
9 |
TEST REPORT Electrical safety according to IEC 60601-1, IEC 62304, Medical Electrical Equipment and EN 957-1, EN 957-6
|
DAkkS accredited and independent testing laboratory |
264 |
10 |
TEST REPORT for EMC Electromagnetic compatibility according to EN 60601-1-2 / EN 55011 and relevant standards
|
DAkkS accredited and independent testing laboratory |
88 |
11 |
Clinical data / Clinical Evaluation Report CER file incl. plan
|
Independent, competent and certified medical technology company |
268 |
12 |
Approval documentation Gate 1 to Gate 4 incl. customer requirements, regulatory requirements, specifications, functional specifications, verification and validation in tabular form – overview
|
h/p/cosmos |
100 |
13 |
Market surveillance Post Market Surveillance PMS plan incl. PMCF & PMS report: 20240619_0539_PMS-Plan incl. PMCF & PMS-Report.pdf |
h/p/cosmos |
70 |
14 |
Periodic Safety Update Report (PSUR)20240611_hpcosmos_treadmills_Periodic Safety Update Report (PSUR)_signed.pdf |
h/p/cosmos |
37 |
Not yet listed in the table above are technical drawings, circuit diagrams, simulations, component data sheets, biocompatibility certificates, software specifications and validations in accordance with EN 62304, service manuals, training certificates, etc., which are of course all available from h/p/cosmos and form part of the audits.
This test report and supporting documentation for compliance with regulatory requirements and standards (including EN ISO 20957-1 and EN ISO 20957-6, the standard for treadmills, including those used in sports) were partly prepared by TÜV SÜD Product Service GmbH (an independent testing laboratory) and, together with the EU Declaration of Conformity for Machinery issued by h/p/cosmos, were regularly checked and evaluated by the Notified Body during the audits of the technical documentation in accordance with the sampling plan.
What does an EU Declaration of Conformity for sports treadmills (which are also machines) look like? Here is a sample: download
What does an EU declaration of conformity for treadmills as medical devices (which are also machines) look like?
Here is a sample: download
Before placing a product on the market, h/p/cosmos prepares detailed technical documentation including a risk management file, clinical evaluation (CER file), usability tests and files, technical test reports on the safety of mechanics, electrics, electromagnetic compatibility, biological compatibility and cyber security, and maintains a certified quality management system, as these are the minimum regulatory requirements for medical devices in this risk class.
After placing the product on the market, we are audited annually (sometimes unannounced) and also submit the TecDoc to the notified body for review.
As part of a post-market plan and PMS post-market surveillance (market observation), we maintain a vigilance system for reportable incidents and regularly produce vigilance reports, manufacturer trend reports and PSUR reports (periodic safety update reports), which we also submit regularly to the notified body for review.
Together, this amounts to many thousands of pages of documentation and, of course, the corresponding effort and costs.
But it's all worth it because it's about the safety of athletes, patients and operators, and therefore also the safety of distributors and manufacturers.
Please also read these overviews of the requirements for manufacturers, importers and distributors:
https://blog.johner-institute.com/regulatory-affairs/medical-device-regulation-mdr/
https://blog.johner-institute.com/regulatory-affairs/distributor/
Summary:
- Motorised treadmills as active medical devices for physiotherapy and/or diagnostic uses must always be classified as machines based on EU machinery directive and as active medical devices of Class IIa or Class IIb in accordance with (EU) 2017/745 MDR Rule 9 and, if applicable, additionally in accordance with sub-rule 10.
- Manufacturers of treadmills must be certified by accredited/certified independent notified bodies in accordance with MDR, and their quality management systems, technical documentation, PSUR reports with PMS market surveillance and clinical data (CER file) must be monitored regularly. Comparison: Just as a person cannot issue themselves with a driving licence in the form of a "self-declaration" without having successfully passed a test (even if they think they can drive a car) and/or a technically skilled person cannot issue themselves with a MOT certificate in a "self-declaration" (even if they think their car is safe), manufacturers and their QM systems, including risk management, clinical data and TecDoc for all Class IIa and/or IIb medical devices, must be certified and monitored annually by independent and accredited bodies.
- According to Article 5(1) of the MDR, a medical device may only be placed on the market or put into service if it complies with the requirements of the MDR. The same applies to requirements for machinery under (EU) 2023/1230.
- Incorrect classification of a medical device and/or non-compliance with the EU Machinery Directive means that the product is not marketable and therefore may not be distributed or placed on the European market. Advertising and selling a non-marketable product is not permitted.
- Violation of the provisions of Articles 13 and 14 MDR and also of the provisions of the EU Machinery Regulation 2023/1230 also constitutes a breach of competition law within the meaning of Section 3a UWG (German Unfair Competition Act).
- Economic operators such as distributors and importers also have a duty to cooperate in checking the manufacturer's documents and are jointly responsible for ensuring that the relevant EU declarations of conformity accompany the products.
- Quote from a state authority: [...] Anyone who intentionally or negligently places a machine or safety component on the market without an EC declaration of conformity or CE marking is acting unlawfully (Section 8 No. 9 ProdSV). The administrative offence can be punished with a fine of up to €100,000.00 (cf. Section 28 (2) Product Safety Act (ProdSG)).
[…] If the supervisory authority determines that a machine is being operated without the required declaration of conformity and CE marking, the employer may face a shutdown order until the machine is brought into compliance with the law.
If an accident occurs on such a machine, criminal investigations may be conducted against both the distributor and the employer.
We strongly advise against placing a machine on the market or putting it into service without the required marking and certification.
LINK 1 (North Rhine-Westphalia State Office for Health and Occupational Safety, Occupational Safety in North Rhine-Westphalia)
LINK 2 (BGHM Professional Association for Wood and Metal) - The CE mark is not a test mark from an independent body, but is affixed by the manufacturer itself to confirm that all applicable regulations in the EU are complied with and that the product meets the basic safety and performance requirements.
- For good reason, there are corresponding regulations and rules in the EU and the respective states that mandate an independent notified body for manufacturers of medium- and high-risk medical devices and also require an EU declaration of conformity and technical documentation for machines.
- The state authorities, regional councils, trade supervisory offices and health authorities of the federal states are responsible for operational market surveillance, can carry out random checks, product tests and inspections at manufacturers, distributors and importers, and can assess complaints and incidents.
The document was created by treadmill specialist Franz Harrer in collaboration with h/p/cosmos management and Regulatory Affairs, authorised signatory and sports scientist Dr. Björn Zimmermann, authorised signatory and PRRC Nadine Schott, and also references many expert opinions.
If you have any questions about regulatory issues or new evidence on this topic, please send an email to: regulatory.affairs@hpcosmos.com
Thank you for your cooperation in the interests of safety!
With cosmic regards
Alexander Böck
Managing Director

